In discussions about side effects of testosterone treatment, prostate cancer and heart disease get the most attention. However, as we have described in several study reports published here, the widespread fear of prostate cancer and heart disease is unfounded and not supported by medical research.
The expected potential side effect of testosterone treatment – which in fact is a therapeutic effect in men with anemia [1-3] – is an increased level of red blood cells, known as erythrocytosis or polycythemia.[4-7] In the context of testosterone treatment, erythrocytosis and polycythemia are used interchangeably to refer to an abnormal increase of red blood cells or hematocrit, which may increase blood viscosity (“blood thickness”).[8, 9]
However, it should be pointed out that technically, erythrocytosis is just red blood cell elevation, while polycythemia involves elevation of red blood cells, white blood cells, and platelets. Using these terms as synonyms can cause confusion. In polycythemia, it is likely the increase in platelets that is the major culprit of blood clots. The clinical significance of a high hematocrit level is unclear, but it may theoretically be associated with an increased risk of thrombosis (blood clots).[4]
Here I summarize the results of an analysis of the effect of different testosterone preparations on hematocrit elevations, published in the journal Sexual Medicine Reviews.[10]
Key Points:
- An expected potential side effect of testosterone treatment is an increased level of red blood cells, which manifests as increases levels of hemoglobin and hematocrit. This “side effect” is actually a desired therapeutic effect in men with anemia.
- It has not been directly proven that testosterone-induced elevations in hematocrit may increase risk of venous thromboembolism.
- Because it is theoretically plausible that high hematocrit levels may increase risk for cardiovascular events – including blood clots – regular monitoring of hematocrit during testosterone therapy is important.
- Testosterone treatment may confer several other beneficial effects that counteract possible risks of high levels of hematocrit.
What IS known:
Hemoglobin is a protein in red blood cells responsible for the delivery of oxygen to tissues. To ensure adequate oxygenation, a sufficient hemoglobin level must be maintained. The amount of hemoglobin in whole blood is expressed in grams per deciliter (g/dL). The normal hemoglobin level is 14 to 18 g/dL for males and 12 to 16 g/dL for females.[11] When the hemoglobin level is low, the patient has anemia. Erythrocytosis is a condition caused by too many red cells, which results in hemoglobin levels above normal, often accompanied by an elevated hematocrit.[11]
Hematocrit measures the volume of red blood cells compared to the total blood volume (red blood cells and plasma); normal hematocrit levels for men is 40 to 54% and for women 36 to 48%.[11] Both hemoglobin and hematocrit are based on whole blood and are therefore dependent on plasma volume. If a patient is dehydrated, the hemoglobin and hematocrit will be higher than if the patient were normovolemic. If the patient is fluid overloaded, values will be lower than their actual level.[11]
Testosterone has a well-documented erythrogenic effect that increases red blood cell production.[1, 12-15]
Testosterone treatment is associated with a dose-dependent increase in hemoglobin and hematocrit levels [16-18]; the increases in hemoglobin and hematocrit are greater in older men than in young men.[18, 19] The effect of testosterone therapy on erythropoiesis may become evident at three months and peaks after approximately twelve months.[20]
An increased hematocrit is associated with increased blood viscosity (“blood thickening”), increased platelet activation/aggregation, decreased venous return and shortened bleeding time.[8, 9, 21-25] Although stimulation of red blood cell production with an ensuing rise in hemoglobin and hematocrit is beneficial for patients with anemia, it is theoretically possible that elevations above the normal range may have unintended consequences.[10, 26]
The potential risk of hematocrit elevations has been suggested by research in patients with polycythemia vera, which showed that a hematocrit target of less than 45% resulted in a significantly lower rate of thrombosis (blood clots) and cardiovascular death than a hematocrit target of 45 – 50%.[27] Polycythemia vera is a slow-growing blood cancer in which the bone marrow produces too many red blood cells. These excess red blood cells thicken the blood, slow down blood flow and cause complications, such as thrombosis, which can lead to a heart attack or stroke.[28] However, several studies have demonstrated that high endogenous testosterone levels are not associated with venous thromboembolism or deep vein thrombosis in healthy men.[29-31] In line with this, there is no undisputable evidence that hematocrit elevation following testosterone therapy increases incidence of either blood clots, heart attack, or stroke in the general population of men.[32-37] Reported cases of venous thromboembolism after testosterone treatment were almost all confined to patients with a previously undiagnosed thrombophilia.[34, 38] Men with thrombophilia are more likely to have the Factor V Leiden mutation, high Factor VIII and high Factor XI than men given testosterone therapy who did not have a venous thromboembolism.[34, 38] This is supported by more recent study which showed that among adult men with low testosterone levels who were at low to moderate baseline risk of deep vein thrombosis or pulmonary embolism – which comprises the large majority of the general population of men – there is no significant association between testosterone replacement therapy and risk of deep vein thrombosis or pulmonary embolism.[37]
Two large pharmacoepidemiological studies have compared the rate of venous thromboembolism in men receiving testosterone treatment versus a control group (men not receiving testosterone treatment).[39, 40] The first study – which I have written about previously “Risk of Blood Clots in Men Receiving Testosterone Therapy” – showed that testosterone treatment is not associated with an increased risk of venous thromboembolism.[39] The second study concluded that starting testosterone treatment is associated with an increased risk of venous thromboembolism during the first 6 months of testosterone treatment, and declines thereafter.[40] A meta-analysis was recently conducted on these two studies.[33] A meta-analysis is a statistical approach to combine the results from multiple studies in an effort to increase statistical power (over individual studies), improve estimates of the effect size and/or to resolve uncertainty when studies disagree.[41, 42] This meta-analysis showed that testosterone treatment is not associated with an increased risk of venous thromboembolism, even when the analysis was confined to the first 6 months of testosterone therapy.[33] Risk of blood clots seems to be more strongly associated with estrogen and estrogen treatment.[43-47] The contribution of estrogen as a causal factor in venous thromboembolism is supported by the World Professional Association for Transgender Health (WPATH), which report that the greatest risk factor of male-to-female estrogen hormone treatment is venous thromboembolism.[45] This is not the case in female-to-male transsexuals, who are treated with testosterone.[45, 47]
What This Analysis Adds
Even though it has not been directly proven that testosterone-induced elevations in hematocrit may increase risk of venous thromboembolism, since it is mechanistically plausible, regular monitoring of hematocrit during testosterone therapy is important.[4-6, 48] In clinical practice, erythrocytosis commonly translates to a hemoglobin level higher than 18.5g/dL or an hematocrit level higher than 52% in men, although the exact cut-off varies between guidelines. The Endocrine Society uses a Hematocrit level higher than 50% as a relative contraindication to the initiation of testosterone therapy and a hematocrit level higher than 54% as a reason to stop testosterone therapy until hematocrit returns to a safer level.[48] The European Association of Urology (EAU) also recommends 54% as the upper safe hematocrit threshold.[4] Other professional societies use hematocrit levels ranging from 52% to 55% as thresholds to modify or discontinue testosterone therapy.[49] All major guidelines strongly recommend measuring hematocrit at baseline, at 3 and 6 months after start of testosterone therapy, and then annually.[4-6, 48] It should be noted that Isolated hematocrit elevations can be the result of insufficient fluid intake on a hot day or dehydration following vigorous exercise. Only repeated measures of hematocrit above 54% should be followed by concomitant administration of aspirin, therapeutic phlebotomy and/or discontinuation of testosterone treatment until hematocrit declines below 54%. After normalization of hematocrit level testosterone treatment may be continued with a reduced dosage.[4-6, 48, 50-52]
Therefore, in clinical practice it is of interest to know which testosterone preparations tend to cause the largest and smallest elevations in hematocrit. The results from the recent analysis published in Sexual Medicine Reviews are summarized in table 1.[10]
Table 1: Varying effect on hematocrit with different testosterone formulations.[10]
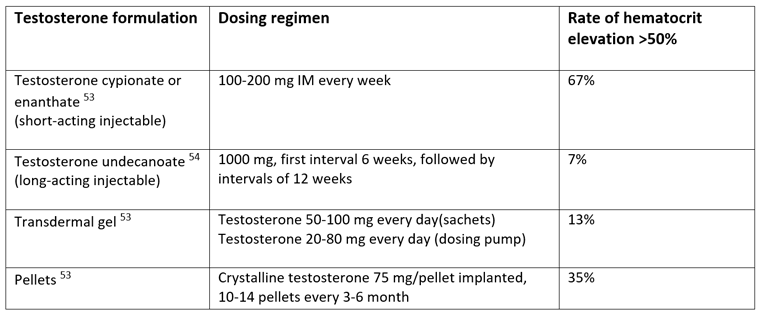
Table modified from Ohlander SJ, Varghese B, Pastuszak AW. Erythrocytosis Following Testosterone Therapy. Sex Med Rev. 2017 [May 16, Epub ahead of print]
The risk of reaching hematocrit >54% is determined by the duration of supraphysiologic testosterone levels, which in turn is determined by testosterone formulation (and hence pharmacokinetics) and dose.[18, 26, 55-57] Short-acting intramuscular testosterone formulations (testosterone cypionate and testosterone enanthate) are associated with the most rapid and marked increases in testosterone levels – and hence hematocrit elevation – with supraphysiologic testosterone levels achieved within days of an injection and a return to baseline after 10 to 14 days, followed by a decrease to sub-physiologic levels within 3 weeks if not re-injected.[26, 58] In contrast, other testosterone formulations that result in a slower and more stable increase in testosterone levels, such as long-acting testosterone undecanoate injections, transdermal gels and pellets, result in a low incidence of erythrocytosis that is dependent on dose and achieved testosterone level, and independent of duration of testosterone therapy.[18, 55, 56] Long-acting testosterone undecanoate maintains stable testosterone levels within the normal range for approximately 12 weeks.[59] Hemoglobin and hematocrit increase initially over the first 3-6 months of treatment but then stabilize and remain within the normal range over the entire treatment period.[60, 61]
A prospective observational study specifically examined adverse effects, including hematocrit elevations, of long-acting testosterone undecanoate; 347 hypogonadal men received a total of 3,022 testosterone undecanoate injections over 3.5 years.[54] Only 14 patients (4%) developed a hematocrit level higher than 52% and 25 patients (7%) developing a hematocrit level higher than 54%.[54] In stark contrast, among men receiving short-acting testosterone enanthate injections formulations, up to 67% experience hematocrit elevations higher than 50%.[53] A previous pharmacokinetic study comparing long-acting testosterone undecanoate (1,000 mg every 6 weeks, followed by 1,000 mg every 9 weeks) and short-acting testosterone enanthate (250 mg every 3 weeks) also showed higher, stable trough testosterone levels for testosterone undecanoate at the time of injections (14.9 to 16.5 nmol/L) compared with testosterone enanthate (<10 nmol/L).[59]
Comments
Almost all previously reported cases of testosterone treatment related venous thromboembolism were seen in patients with a previously undiagnosed thrombophilia.[34, 38] Considering the low annual incidence of venous thromboembolism in the general population – 48 to 120 per 100 000 people [62-64] – fear of venous thromboembolism should not preclude the large majority of suffering hypogonadal men from receiving testosterone therapy and its well documented health benefits. The prevalence of hereditary thrombophilia has been reported to be 9% in a healthy population.[65] While screening for thrombophilia before starting testosterone therapy is warranted for men with a history or family history of thrombosis, data do not justify universal screening because the absolute risk remains low.[66]
The risk of elevated hematocrit seen in patients with polycythemia vera cannot be extrapolated to hematocrit elevations seen during testosterone therapy in men without blood cancer or genetic mutations. Data suggest that testosterone therapy has effects that may counteract the potentially increased risk of venous thromboembolism. For example, testosterone therapy is one of the few treatments that reduces lipoprotein(a) [Lp(a)] levels, in the impressive range of 20-59%.[67-69] In line with this, suppression of endogenous testosterone in young healthy men significantly increases Lp(a) levels, up to 40-60% above baseline.[70] It has been suggested that Lp(a) may be a risk factor for venous thromboembolism.[71-73] Mendelian randomization studies did not confirm an association between Lp(a) with the risk of venous thrombosis in the general population studies.[74, 75] However, a meta-analysis of eight studies in pediatric populations showed that elevated Lp(a) levels are associated with a 4.5-fold (odds ratio 4.50) increased risk of first onset venous thromboembolism.[76] Lp(a) may not necessarily be prothrombotic, but may tilt the balance towards thrombosis due to the potential loss of fibrinolytic activity.[77] Interestingly, the Mendelian randomization studies which did not find an association between Lp(a) with the risk of venous thrombosis did show an association between Lp(a) and factor V Leiden (which increases risk of blood clotting).[74, 75] Although further study is needed, this suggests that elevated Lp(a) may need a second underlying factor to generate a thrombus and that reductions in Lp(a) levels may reduce the overall thrombotic propensity.[77]
A second reason that elevations in hematocrit with testosterone treatment may not be inherently dangerous is that low testosterone levels are associated with higher levels of prothrombotic factors in men, regardless of age, obesity, body fat distribution, and related metabolic parameters.[78]
Finally, an experimental study suggests that there are adaptive physiological mechanisms that restore whole-blood viscosity to normal during prolonged testosterone administration.[79] This study comprehensively assessed the effects of testosterone on whole-blood viscosity, plasma viscosity, and erythrocyte deformability, and compared sex differences after short-term as well as longer-term testosterone treatments.[79] Results showed that long-term testosterone treatment did not adversely affect whole-blood viscosity or plasma viscosity in adult mice, even when supraphysiologic testosterone levels were reached. Interestingly, erythrocyte deformability was increased after long-term high-dose testosterone treatment. In contrast, short-term treatment with high-dose testosterone transiently raised whole-blood viscosity in association with increased hematocrits in female and castrated male mice.[79] The increased erythrocyte deformability may offset whole-blood viscosity to a much lower level than that predicted from exceptionally high hematocrits.[79, 80] Notably, testosterone treatment in hypogonadal men has been shown to improve erythrocyte membrane composition and fluidity [81], which likely improves blood rheology and contributes to a reduced thrombosis risk.[82] Testosterone also has vasodilator and anti-atherosclerotic effects that in addition may explain the lack of increase in cardiovascular events with elevated hematocrit during testosterone therapy.[83-85]
Support for the better tolerance to hematocrit elevations during testosterone treatment with testosterone undecanoate comes from a study where one patient showed constantly elevated hematocrit values when treated with testosterone enanthate.[86] This patient showed no elevated hematocrit levels in the 27 month follow-up study when receiving testosterone undecanoate treatment.[86] This suggests that men who experience marked hematocrit elevations with short-acting injectable testosterone formulations may be better able to tolerate treatment with testosterone undecanoate.
A notable study retrospectively reviewed the charts of 217 hypogonadal men older than 65 years who were treated with testosterone therapy, to determine the prevalence of thrombotic events and all-cause mortality.[36] There was increased all-cause mortality in hypogonadal men not treated with testosterone compared to men who received testosterone treatment.[36] There was no difference in incidence of myocardial infarction, transient ischemic attack, stroke or deep vein thrombosis/pulmonary embolism between patients treated with testosterone and hypogonadal men not treated with testosterone.[36] In the TEAAM (Testosterone’s Effects on Atherosclerosis Progression in Aging Men) trial, of 155 men who were treated with testosterone for 3 years, 13 men (8%) experienced hematocrit greater than 54%. Hence, the incidence of large hematocrit elevations during long-term testosterone therapy is small. Considering the significant reduction in mortality seen in several studies in testosterone treated men compared to non-treated men [36, 87-90], the small number of men experiencing hematocrit elevations reaching 54% – the consequences of which are still unproven [32-36] – should not deter physicians from prescribing testosterone treatment to suffering hypogonadal men.
References:
1. Shahani S, Braga-Basaria M, Maggio M, Basaria S. Androgens and erythropoiesis: past and present. J Endocrinol Invest. 2009;32(8):704-716.
2. Zhang LT, Shin YS, Kim JY, Park JK. Could Testosterone Replacement Therapy in Hypogonadal Men Ameliorate Anemia, a Cardiovascular Risk Factor? An Observational, 54-Week Cumulative Registry Study. J Urol. 2016;195(4 Pt 1):1057-1064.
3. Roy CN, Snyder PJ, Stephens-Shields AJ, et al. Association of Testosterone Levels With Anemia in Older Men: A Controlled Clinical Trial. JAMA internal medicine. 2017;177(4):480-490.
4. Dohle GR, Arver S, Bettocchi C, Jones TH, Kliesch S, Punab M. 2016 EAU Guidelines on Male Hypogonadism, available at http://uroweb.org/wp-content/uploads/EAU-Guidelines-Male-Hypogonadism-2016.pdf (accessed January 11, 2017).
5. Dean JD, McMahon CG, Guay AT, et al. The International Society for Sexual Medicine’s Process of Care for the Assessment and Management of Testosterone Deficiency in Adult Men. The journal of sexual medicine. 2015;12(8):1660-1686.
6. Morales A, Bebb RA, Manjoo P, et al. Diagnosis and management of testosterone deficiency syndrome in men: clinical practice guideline. Appendix available at: http://www.cmaj.ca/content/suppl/2015/10/26/cmaj.150033.DC1/15-0033-1-at.pdf (accessed Jan 10, 2016). CMAJ. 2015;187(18):1369-1377.
7. Calof OM, Singh AB, Lee ML, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60(11):1451-1457.
8. Baskurt OK, Meiselman HJ. Blood rheology and hemodynamics. Semin Thromb Hemost. 2003;29(5):435-450.
9. Wells RE, Jr., Merrill EW. Influence of flow properties of blood upon viscosity-hematocrit relationships. J Clin Invest. 1962;41:1591-1598.
10. Ohlander SJ, Varghese B, Pastuszak AW. Erythrocytosis Following Testosterone Therapy. Sex Med Rev. 2017.
11. Billett HH. Hemoglobin and Hematocrit. In: Walker HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston1990.
12. Gardner FH, Pringle JC, Jr. Androgens and erythropoiesis: I. preliminary clinical observations. Arch Intern Med. 1961;107(6):846-862.
13. Kennedy BJ. Stimulation of erythropoiesis by androgenic hormones. Ann Intern Med. 1962;57:917-936.
14. Naets JP, Wittek M. THE MECHANISM OF ACTION OF ANDROGENS ON ERYTHROPOIESIS*. Ann N Y Acad Sci. 1968;149(1):366-376.
15. Shahidi NT. Androgens and Erythropoiesis. N Engl J Med. 1973;289(2):72-80.
16. Bhasin S, Woodhouse L, Casaburi R, et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281(6):E1172-1181.
17. Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90(2):678-688.
18. Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab. 2008;93(3):914-919.
19. Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90(2):678-688.
20. Saad F, Aversa A, Isidori AM, Zafalon L, Zitzmann M, Gooren L. Onset of effects of testosterone treatment and time span until maximum effects are achieved. Eur J Endocrinol. 2011;165(5):675-685.
21. Hellem AJ, Borchgrevink CF, Ames SB. The role of red cells in haemostasis: the relation between haematocrit, bleeding time and platelet adhesiveness. Br J Haematol. 1961;7:42-50.
22. Guyton AC, Richardson TQ. Effect of hematocrit on venous return. Circ Res. 1961;9:157-164.
23. Litvinov RI, Weisel JW. Role of red blood cells in haemostasis and thrombosis. ISBT Sci Ser. 2017;12(1):176-183.
24. Lowe GDO. Blood Rheology, Blood Flow and Disease. In: Hwang NHC, Turitto VT, Yen MRT, eds. Advances in Cardiovascular Engineering. Boston, MA: Springer US; 1992:103-107.
25. Lowe GD, Forbes CD. Platelet aggregation, haematocrit, and fibrinogen. Lancet. 1985;1(8425):395-396.
26. Rhoden EL, Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med. 2004;350(5):482-492.
27. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22-33.
28. Spivak JL. Polycythemia vera: myths, mechanisms, and management. Blood. 2002;100(13):4272-4290.
29. Svartberg J, Braekkan SK, Laughlin GA, Hansen JB. Endogenous sex hormone levels in men are not associated with risk of venous thromboembolism: the Tromso study. Eur J Endocrinol. 2009;160(5):833-838.
30. Holmegard HN, Nordestgaard BG, Schnohr P, Tybjaerg-Hansen A, Benn M. Endogenous sex hormones and risk of venous thromboembolism in women and men. Journal of thrombosis and haemostasis : JTH. 2014;12(3):297-305.
31. Mumoli N, Cei M, Giorgi Pierfranceschi M, Brondi B, Vitale J, Dentali F. Endogenous sex hormone levels in men with unprovoked deep-vein thrombosis. Thromb Haemost. 2015;114(2):438-439.
32. Ohlander SJ, Varghese B, Pastuszak AW. Erythrocytosis Following Testosterone Therapy. Sex Med Rev, [May 16, Epub ahead of print]. 2017.
33. Corona G, Dicuio M, Rastrelli G, et al. Testosterone treatment and cardiovascular and venous thromboembolism risk: what is ‘new’? J Investig Med. 2017.
34. Glueck CJ, Wang P. Testosterone therapy, thrombosis, thrombophilia, cardiovascular events. Metabolism. 2014;63(8):989-994.
35. Jones SD, Jr., Dukovac T, Sangkum P, Yafi FA, Hellstrom WJ. Erythrocytosis and Polycythemia Secondary to Testosterone Replacement Therapy in the Aging Male. Sex Med Rev. 2015;3(2):101-112.
36. Ramasamy R, Scovell J, Mederos M, Ren R, Jain L, Lipshultz L. Association Between Testosterone Supplementation Therapy and Thrombotic Events in Elderly Men. Urology. 2015;86(2):283-285.
37. Sharma R, Oni OA, Chen G, et al. Association Between Testosterone Replacement Therapy and the Incidence of DVT and Pulmonary Embolism: A Retrospective Cohort Study of the Veterans Administration Database. Chest. 2016;150(3):563-571.
38. Glueck CJ, Prince M, Patel N, et al. Thrombophilia in 67 Patients With Thrombotic Events After Starting Testosterone Therapy. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2016;22(6):548-553.
39. Baillargeon J, Urban RJ, Morgentaler A, et al. Risk of Venous Thromboembolism in Men Receiving Testosterone Therapy. Mayo Clin Proc. 2015;90(8):1038-1045.
40. Martinez C, Suissa S, Rietbrock S, et al. Testosterone treatment and risk of venous thromboembolism: population based case-control study. BMJ. 2016;355:i5968.
41. Haidich AB. Meta-analysis in medical research. Hippokratia. 2010;14(Suppl 1):29-37.
42. Stroup DF, Thacker SB. Meta-Analysis in Epidemiology. Encyclopedia of Biostatistics: John Wiley & Sons, Ltd; 2005.
43. Group ECW. Venous thromboembolism in women: a specific reproductive health risk. Hum Reprod Update. 2013;19(5):471-482.
44. Rott H. Prevention and treatment of venous thromboembolism during HRT: current perspectives. International journal of general medicine. 2014;7:433-440.
45. Coleman E, Bockting W, Botzer M, et al. Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7. International Journal of Transgenderism. 2012;13(4):165-232.
46. Meriggiola MC, Jannini EA, Lenzi A, Maggi M, Manieri C. Endocrine treatment of transsexual persons: an Endocrine Society Clinical Practice Guideline: commentary from a European perspective. Eur J Endocrinol. 2010;162(5):831-833.
47. Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, et al. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2009;94(9):3132-3154.
48. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559.
49. Wang C, Nieschlag E, Swerdloff RS, et al. ISA, ISSAM, EAU, EAA and ASA recommendations: investigation, treatment and monitoring of late-onset hypogonadism in males. The aging male : the official journal of the International Society for the Study of the Aging Male. 2009;12(1):5-12.
50. Aversa A, Morgentaler A. The practical management of testosterone deficiency in men. Nature reviews Urology. 2015;12(11):641-650.
51. Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. The aging male : the official journal of the International Society for the Study of the Aging Male. 2015;18(1):5-15.
52. Aversa A, Morgentaler A. The practical management of testosterone deficiency in men. Nature reviews Urology. 2015.
53. Pastuszak AW, Gomez LP, Scovell JM, Khera M, Lamb DJ, Lipshultz LI. Comparison of the Effects of Testosterone Gels, Injections, and Pellets on Serum Hormones, Erythrocytosis, Lipids, and Prostate-Specific Antigen. Sex Med. 2015;3(3):165-173.
54. Middleton T, Turner L, Fennell C, et al. Complications of injectable testosterone undecanoate in routine clinical practice. Eur J Endocrinol. 2015;172(5):511-517.
55. Dobs AS, Meikle AW, Arver S, Sanders SW, Caramelli KE, Mazer NA. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab. 1999;84(10):3469-3478.
56. Ip FF, di Pierro I, Brown R, Cunningham I, Handelsman DJ, Liu PY. Trough serum testosterone predicts the development of polycythemia in hypogonadal men treated for up to 21 years with subcutaneous testosterone pellets. Eur J Endocrinol. 2010;162(2):385-390.
57. Jockenhovel F, Vogel E, Reinhardt W, Reinwein D. Effects of various modes of androgen substitution therapy on erythropoiesis. Eur J Med Res. 1997;2(7):293-298.
58. Bhasin S, Bagatell CJ, Bremner WJ, et al. Issues in testosterone replacement in older men. J Clin Endocrinol Metab. 1998;83(10):3435-3448.
59. Schubert M, Minnemann T, Hubler D, et al. Intramuscular testosterone undecanoate: pharmacokinetic aspects of a novel testosterone formulation during long-term treatment of men with hypogonadism. J Clin Endocrinol Metab. 2004;89(11):5429-5434.
60. Saad F, Kamischke A, Yassin A, et al. More than eight years’ hands-on experience with the novel long-acting parenteral testosterone undecanoate. Asian journal of andrology. 2007;9(3):291-297.
61. Yassin AA, Haffejee M. Testosterone depot injection in male hypogonadism: a critical appraisal. Clinical interventions in aging. 2007;2(4):577-590.
62. Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ, 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158(6):585-593.
63. Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med. 2006;21(7):722-727.
64. Ruppert A, Lees M, Steinle T. Clinical burden of venous thromboembolism. Curr Med Res Opin. 2010;26(10):2465-2473.
65. Bombeli T, Basic A, Fehr J. Prevalence of hereditary thrombophilia in patients with thrombosis in different venous systems. Am J Hematol. 2002;70(2):126-132.
66. Wu O, Robertson L, Twaddle S, et al. Screening for thrombophilia in high-risk situations: systematic review and cost-effectiveness analysis. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study. Health Technol Assess. 2006;10(11):1-110.
67. Berglund L, Carlstrom K, Stege R, et al. Hormonal regulation of serum lipoprotein (a) levels: effects of parenteral administration of estrogen or testosterone in males. J Clin Endocrinol Metab. 1996;81(7):2633-2637.
68. Marcovina SM, Lippi G, Bagatell CJ, Bremner WJ. Testosterone-induced suppression of lipoprotein(a) in normal men; relation to basal lipoprotein(a) level. Atherosclerosis. 1996;122(1):89-95.
69. Zmunda JM, Thompson PD, Dickenson R, Bausserman LL. Testosterone decreases lipoprotein(a) in men. Am J Cardiol. 1996;77(14):1244-1247.
70. von Eckardstein A, Kliesch S, Nieschlag E, Chirazi A, Assmann G, Behre HM. Suppression of endogenous testosterone in young men increases serum levels of high density lipoprotein subclass lipoprotein A-I and lipoprotein(a). J Clin Endocrinol Metab. 1997;82(10):3367-3372.
71. von Depka M, Nowak-Gottl U, Eisert R, et al. Increased lipoprotein (a) levels as an independent risk factor for venous thromboembolism. Blood. 2000;96(10):3364-3368.
72. Sofi F, Marcucci R, Abbate R, Gensini GF, Prisco D. Lipoprotein (a) and venous thromboembolism in adults: a meta-analysis. Am J Med. 2007;120(8):728-733.
73. Grifoni E, Marcucci R, Ciuti G, et al. The thrombophilic pattern of different clinical manifestations of venous thromboembolism: a survey of 443 cases of venous thromboembolism. Semin Thromb Hemost. 2012;38(2):230-234.
74. Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Genetic evidence that lipoprotein(a) associates with atherosclerotic stenosis rather than venous thrombosis. Arterioscler Thromb Vasc Biol. 2012;32(7):1732-1741.
75. Helgadottir A, Gretarsdottir S, Thorleifsson G, et al. Apolipoprotein(a) genetic sequence variants associated with systemic atherosclerosis and coronary atherosclerotic burden but not with venous thromboembolism. J Am Coll Cardiol. 2012;60(8):722-729.
76. Young G, Albisetti M, Bonduel M, et al. Impact of inherited thrombophilia on venous thromboembolism in children: a systematic review and meta-analysis of observational studies. Circulation. 2008;118(13):1373-1382.
77. Tsimikas S. A Test in Context: Lipoprotein(a): Diagnosis, Prognosis, Controversies, and Emerging Therapies. J Am Coll Cardiol. 2017;69(6):692-711.
78. De Pergola G, De Mitrio V, Sciaraffia M, et al. Lower androgenicity is associated with higher plasma levels of prothrombotic factors irrespective of age, obesity, body fat distribution, and related metabolic parameters in men. Metabolism. 1997;46(11):1287-1293.
79. Guo W, Bachman E, Vogel J, et al. The effects of short-term and long-term testosterone supplementation on blood viscosity and erythrocyte deformability in healthy adult mice. Endocrinology. 2015;156(5):1623-1629.
80. Vogel J, Kiessling I, Heinicke K, et al. Transgenic mice overexpressing erythropoietin adapt to excessive erythrocytosis by regulating blood viscosity. Blood. 2003;102(6):2278-2284.
81. Angelova P, Momchilova A, Petkova D, Staneva G, Pankov R, Kamenov Z. Testosterone replacement therapy improves erythrocyte membrane lipid composition in hypogonadal men. The aging male : the official journal of the International Society for the Study of the Aging Male. 2012;15(3):173-179.
82. Simmonds MJ, Meiselman HJ, Baskurt OK. Blood rheology and aging. Journal of geriatric cardiology : JGC. 2013;10(3):291-301.
83. Jones TH, Saad F. The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process. Atherosclerosis. 2009;207(2):318-327.
84. Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol. 2013;217(3):R47-71.
85. Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013;217(3):R25-45.
86. Minnemann T, Schubert M, Freude S, et al. Comparison of a new long-acting testosterone undecanoate formulation vs testosterone enanthate for intramuscular androgen therapy in male hypogonadism. J Endocrinol Invest. 2008;31(8):718-723.
87. Traish AM, Haider A, Haider KS, Doros G, Saad F. Long-Term Testosterone Therapy Improves Cardiometabolic Function and Reduces Risk of Cardiovascular Disease in Men with Hypogonadism. J Cardiovasc Pharmacol Ther. 2017:1074248417691136.
88. Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36(40):2706-2715.
89. Muraleedharan V, Marsh H, Kapoor D, Channer KS, Jones TH. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169(6):725-733.
90. Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97(6):2050-2058.