In discussions about women’s health, the spotlight is centered on estrogen. The almost exclusive focus on the role of ovarian estrogens when dealing with menopause issues has overshadowed the importance of other hormones. DHEA is one of them. This article will explain why DHEA is important for women’s health and wellbeing, and present studies on DHEA supplementation and its beneficial effects in peri- and post-menopausal women.
DHEA(S) levels across the lifespan
DHEA (dehydroepiandrosterone) is a pro-hormone that is produced in the adrenals and gets converted to testosterone and estrogen in many tissues. In the blood stream DHEA circulates primarily in its sulfated form DHEA-S.[1-3] The notation DHEA(S) refers to both DHEA and DHEA-S.
The sulfated from of DHEA has a longer half-life in the blood and its levels remain stable throughout the day, are not altered significantly by the menstrual cycle.[1] When getting a blood test for DHEA, the fraction that is routinely measured is therefore DHEA-S. In response to metabolic demand, DHEA-S is rapidly converted back to DHEA (e.g. is hydrolyzed to DHEA by sulfatases).
In contrast to men, whose testosterone production decline gradually with age [2, 3], women face a relatively abrupt cessation of estrogen production at menopause. In both men and women, DHEA levels decrease approximately 80% between ages 25 and 75 year.[4, 5] The most dramatic fall in blood levels of DHEA(S) already occurs between the ages of 20-30 and 40 -50 years, when levels drop by 60-70%.[4, 6, 7] Sine DHEA is transformed to both androgens and estrogens in tissues, such a fall in blood levels of DHEA(S) explains why women at menopause are not only lacking estrogens but are also likely to have been deprived of testosterone for several years.[6] This explains why the beneficial effects of DHEA supplementation are greater and more noticeable in post-menopausal women than in men.[8] Figure 1 illustrates the dramatic difference in sex hormone levels (estrogen and testosterone) between men and post-menopausal women.
Figure 1: Contribution of the gonads (testis in men, ovaries in women) and DHEA (from the adrenals in both sexes) to the total pool of androgens (primarily testosterone) and estrogens in men (A) and women (B) with age.
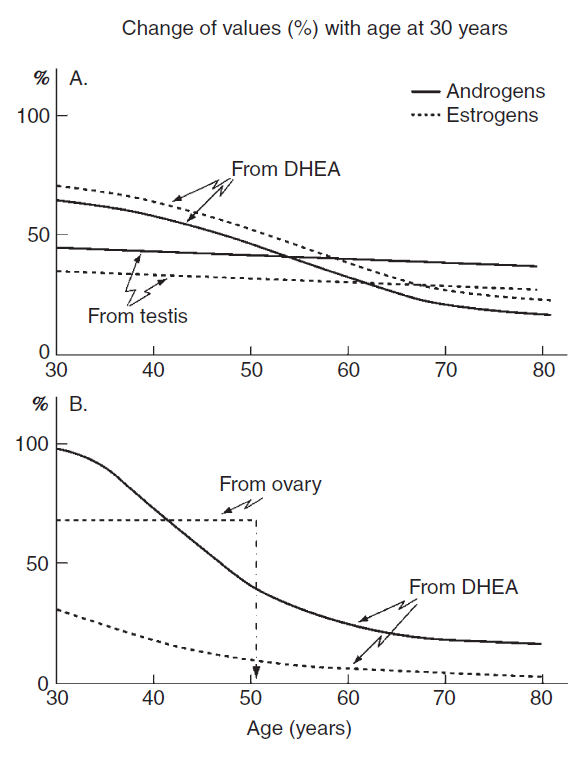
From Labrie F: DHEA, important source of sex steroids in men and even more in women. Progress in Brain Research, Vol. 182
What follows below is an outline of the beneficial effects of DHEA supplementation which have been demonstrated in studies on post-menopausal women. It should be noted that while many studies presented below used a supplemental DHEA dose of 50 mg/day, in clinical practice a dose of 10-25 mg may be more appropriate for some women. When looking at dose-response effects in studies, it is important to remember that there is great inter-individual variability in both baseline DHEA(S) and sex hormone (estrogen and testosterone) levels, which will affect the response to DHEA supplementation. The ideal DHEA dose for every individual woman therefore should be established by blood testing under the direction of a doctor who knows how to tailor hormone dosages based on every individual woman’s clinical picture.
Can DHEA supplementation help women suffering from menopausal symptoms?
Menopausal symptoms, especially hot flashes, shown a great deal of variability in frequency and severity between women.[9-11] Aside lifestyle factors (for more info, see Menopause HRT basics), one major contribution to this inter-individual variability is the wide distribution of DHEA levels and its metabolites, estrogen and testosterone.[12, 13]
A notable study measured blood levels of DHEA, estrogen and testosterone in postmenopausal women aged 42 to 74 years. It was found that the lowest and highest recorded levels of DHEA varied 8-fold between women.[13] The inter-individual variability in testosterone and estrogen levels was even greater, 10-fold for testosterone and 12-fold estrogen.[13]
Support for the notion that the large inter-individual variability in DHEA levels in women is a main reason some women don’t suffer bothersome menopause symptoms, comes from DHEA supplementation studies. Oral supplementation with DHEA improves menopausal symptoms and mid-life depression in peri- and post-menopausal women [14-17], and intra-vaginal DHEA administration (Prasterone) effectively reverses vaginal atrophy and related sexual symptoms in post-menopausal women.[18]
DHEA supplementation provides benefits for both early and late postmenopausal women. One study treated early (50-55 years and late postmenopausal women (60-65 years) with oral DHEA 50 mg/day for 6 months.[15] Treatment with DHEA was associated with a progressive improvement of the menopausal symptoms (both physical and psychological) in all groups, with major effects on vasomotor symptoms in the early postmenopausal women.[15] In addition, cortisol levels progressively decreased in all groups. Interestingly, levels of allopregnanolone and beta-endorphin increased progressively and significantly in all women, each reaching values three times higher than baseline.[15] Several other studies also showed that long-term (6-12 months) DHEA supplementation significantly boosts allopregnanolone and beta-endorphin levels in post-menopausal.[17, 19-21]
Allopregnanolone is a neurosteroid and beta-endorphin is a neuropeptide; both are crucial in the modulation of mood, memory and wellbeing.[22-29] Neurosteroids are important for regeneration and repair systems in the brain, and among this class of molecules, allopregnanolone is been investigated for its role to promote regeneration in both the central and peripheral nervous systems.[22] In experimental studies allopregnanolone has been found to induce regeneration of brain cells in aged mice and mice with Alzheimer disease, accompanied by restoration of learning and memory function.[22]
Reduced levels of allopregnanolone in the blood or cerebrospinal fluid are associated with depression, anxiety disorders, premenstrual dysphoric disorder, and impulsive aggression.[26, 30] Allopregnanolone binds to the GABAA receptor and thereby exerts strong anxiolytic effects.[31] It is interesting that benzodiazepine drugs (which are prescribed for anxiety, insomnia, relaxation etc.) also exert their effects by binding to the GABAA receptor.[32] Thus, one can speculate that the beneficial effects of DHEA supplementation on mood and wellbeing in postmenopausal women, and alleviation of menopausal symptoms, is partly due to its ability to elevate allopregnanolone levels.
Note:
Premenstrual dysphoric disorder (PMDD) is a condition in which a woman has severe depression symptoms, irritability, and tension before menstruation. The symptoms of PMDD are more severe than those seen with premenstrual syndrome (PMS). Because reduced levels of allopregnanolone are seen in women with premenstrual dysphoric disorder, and because DHEA supplementation increases allopregnanolone levels, it is likely that DHEA supplementation may alleviate premenstrual symptoms as well.
Beta-endorphin is an endogenous opioid released from the pituitary gland.[33] Endorphins regulate mood and emotional responses, and induce analgesia, euphoria, mood state changes, and play a role in the reward system in the brain.[34, 35] They resemble the opiates (pain relieving drugs) in their ability to produce analgesia and a sense of well-being.
DHEA vs. traditional HRT
DHEA supplementation alleviates menopausal symptoms to a similar degree as is seen with traditional estrogen HRT.[14, 19, 36] A study that compared 1 year therapy with traditional estrogen therapy (CEE) 0.625 mg/day or DHEA 25 mg/day found that DHEA was as effective as CEE in alleviating menopausal symptoms, without causing side effects seen with CEE (headache and nausea).[36] Another study comparing supplementation of 50 mg DHEA/day with transdermal estradiol 50 micrograms/patch per day also found similar and progressive improvement in menopausal symptoms between treatments.[19]
This effectiveness of DHEA supplementation is very good news for suffering women who have contra-indications for traditional estrogen/progesterone HRT (eg. active cancer or a family history of breast cancer), or who don’t want to take HRT.
DHEA dose considerations
When supplementing with a lower dose, 25 mg DHEA per day, increases in estradiol and testosterone levels are 20% to 45% lower respectively, than with the 50 mg DHEA per day.[15, 17] It should be noted the increase in blood levels of DHEA(S), estradiol, and testosterone is lower and less rapid with a 25 mg/day dose compared to a 50 mg/day dose. This has to be taken into consideration when starting DHEA supplementation as it will impact the time course for onset of benefits.
Also, in these studies, while the 50 mg DHEA per day dose tripled levels of both allopregnanolone and beta-endorphin [15], the lower DHEA dose, 25 mg/day only increased levels of allopregnanolone and beta-endorphin two-fold.[17] However, the lower dose was still reported to be effective for relief of menopausal symptoms in some women.[14, 17, 36] As stated above, this underscores the importance of tailoring the DHEA dose to each individual woman and her health status.
Summary
DHEA is especially important for women because of the relatively abrupt cessation of estrogen production with menopause. DHEA supplementation is a natural way to restore physiological levels of estrogen, as well as testosterone which also drops with age, in women.
Notably, DHEA supplementation can alleviate menopausal symptoms as effectively as traditional estrogen/progesterone hormone replacement therapy, without the side effects (headache and nausea) and potential health risks seen with traditional HRT (for more info on health risks with HRT, see What exactly did the notorious WHI (Women’s Health Initiative) studies show?)
In addition, DHEA supplementation confers significant health benefits beyond mere relief of menopausal symptoms. Notable are its beneficial effects on the bone, vagina, skin and prevention of breast cancer. For more info, see DHEA – specific health benefits for menopausal women
References:
1. Baulieu, E.E., et al., An Adrenal-Secreted “Androgen”: Dehydroisoandrosterone Sulfate. Its Metabolism and a Tentative Generalization on the Metabolism of Other Steroid Conjugates in Man. Recent Prog Horm Res, 1965. 21: p. 411-500.
2. Feldman, H.A., et al., Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab, 2002. 87(2): p. 589-98.
3. Harman, S.M., et al., Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab, 2001. 86(2): p. 724-31.
4. Orentreich, N., et al., Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab, 1984. 59(3): p. 551-5.
5. Orentreich, N., et al., Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J Clin Endocrinol Metab, 1992. 75(4): p. 1002-4.
6. Labrie, F., et al., Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab, 1997. 82(8): p. 2396-402.
7. Migeon, C.J., et al., Dehydroepiandrosterone and androsterone levels in human plasma: effect of age and sex; day-to-day and diurnal variations. J Clin Endocrinol Metab, 1957. 17(9): p. 1051-62.
8. Labrie, F., DHEA, important source of sex steroids in men and even more in women. Prog Brain Res, 2010. 182: p. 97-148.
9. Utian, W.H., Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes, 2005. 3: p. 47.
10. Hemminki, E., et al., Variability of bothersome menopausal symptoms over time–a longitudinal analysis using the Estonian postmenopausal hormone therapy trial (EPHT). BMC Womens Health, 2012. 12: p. 44.
11. Gold, E.B., et al., Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol, 2000. 152(5): p. 463-73.
12. Labrie, F., Impact of circulating dehydroepiandrosterone on androgen formation in women. Menopause, 2011. 18(5): p. 471-3.
13. Labrie, F., C. Martel, and J. Balser, Wide distribution of the serum dehydroepiandrosterone and sex steroid levels in postmenopausal women: role of the ovary? Menopause, 2011. 18(1): p. 30-43.
14. Genazzani, A.R., et al., Effect of 1-year, low-dose DHEA therapy on climacteric symptoms and female sexuality. Climacteric, 2011. 14(6): p. 661-8.
15. Stomati, M., et al., Six-month oral dehydroepiandrosterone supplementation in early and late postmenopause. Gynecol Endocrinol, 2000. 14(5): p. 342-63.
16. Schmidt, P.J., et al., Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch Gen Psychiatry, 2005. 62(2): p. 154-62.
17. Genazzani, A.D., et al., Long-term low-dose dehydroepiandrosterone oral supplementation in early and late postmenopausal women modulates endocrine parameters and synthesis of neuroactive steroids. Fertil Steril, 2003. 80(6): p. 1495-501.
18. Labrie, F., et al., Intravaginal dehydroepiandrosterone (Prasterone), a physiological and highly efficient treatment of vaginal atrophy. Menopause, 2009. 16(5): p. 907-22.
19. Stomati, M., et al., Endocrine, neuroendocrine and behavioral effects of oral dehydroepiandrosterone sulfate supplementation in postmenopausal women. Gynecol Endocrinol, 1999. 13(1): p. 15-25.
20. Genazzani, A.R., et al., Long-term low-dose oral administration of dehydroepiandrosterone modulates adrenal response to adrenocorticotropic hormone in early and late postmenopausal women. Gynecol Endocrinol, 2006. 22(11): p. 627-35.
21. Pluchino, N., et al., One-year therapy with 10mg/day DHEA alone or in combination with HRT in postmenopausal women: effects on hormonal milieu. Maturitas, 2008. 59(4): p. 293-303.
22. Brinton, R.D., Neurosteroids as regenerative agents in the brain: therapeutic implications. Nat Rev Endocrinol, 2013. 9(4): p. 241-50.
23. Maninger, N., et al., Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol, 2009. 30(1): p. 65-91.
24. Melcangi, R.C. and G.C. Panzica, Allopregnanolone: state of the art. Prog Neurobiol, 2014. 113: p. 1-5.
25. Robel, P. and E.E. Baulieu, Neurosteroids Biosynthesis and function. Trends Endocrinol Metab, 1994. 5(1): p. 1-8.
26. Schule, C., C. Nothdurfter, and R. Rupprecht, The role of allopregnanolone in depression and anxiety. Prog Neurobiol, 2014. 113: p. 79-87.
27. Mellon, S.H., Neurosteroids: biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab, 1994. 78(5): p. 1003-8.
28. Majewska, M.D., Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol, 1992. 38(4): p. 379-95.
29. Baulieu, E.E., Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Prog Horm Res, 1997. 52: p. 1-32.
30. Backstrom, T., et al., Allopregnanolone and mood disorders. Prog Neurobiol, 2014. 113: p. 88-94.
31. Brot, M.D., et al., The anxiolytic-like effects of the neurosteroid allopregnanolone: interactions with GABA(A) receptors. Eur J Pharmacol, 1997. 325(1): p. 1-7.
32. Hanson, S.M. and C. Czajkowski, Structural mechanisms underlying benzodiazepine modulation of the GABA(A) receptor. J Neurosci, 2008. 28(13): p. 3490-9.
33. Benarroch, E.E., Endogenous opioid systems: current concepts and clinical correlations. Neurology, 2012. 79(8): p. 807-14.
34. Bodnar, R.J., Endogenous opiates and behavior: 2012. Peptides, 2013. 50: p. 55-95.
35. Harber, V.J. and J.R. Sutton, Endorphins and exercise. Sports Med, 1984. 1(2): p. 154-71.
36. Gupta, B., et al., A Comparative Study of CEE, Tibolone, and DHEA as Hormone Replacement Therapy for Surgical Menopause. J Obstet Gynaecol India, 2013. 63(3): p. 194-8.